Case Report
Submitral Ventricular Pseudoaneurysm: Unusual and Late Complication of Cardiac Surgery

Marzia Cottini1*, Amedeo Pergolini1, Giordano Zampi2, Vitaliano Buffa3, Paolo Giuseppe Pino1, Federico Ranocchi1, Riccardo Gherli1, De Marco Marina1, Carlo Contento1, Myriam Lo Presti1 and Francesco Musumeci1
1Department of Cardiovascular Science, Cardiac Surgery Unit and Heart Transplantation Center, “S. Camillo-Forlanini” Hospital, 00149 Rome, Italy
2Department of Heart and Vessels, Cardiac Unit, “Belcolle Hospital”, 01100 Viterbo, Italy
3Department of Cardiovascular Science, Radiology Unit, “S. Camillo-Forlanini” Hospital, Rome, Italy
*Address for Correspondence: Marzia Cottini, MD, Department of Heart and Vessels, Cardiac Surgery Unit, “S.Camillo-Forlanini” Hospital, Circonvallazione Gianicolense 87, 00149, Rome, Italy, Tel:+39-347-3245331; Fax:+39-06-58704511; Email: [email protected]
Dates: Submitted: 12 December 2016; Approved: 19 January 2017; Published: 21 January 2017
How to cite this article: Cottini M, Pergolini A, Zampi G, Buffa V, Pino PG, et al. Submitral Ventricular Pseudoaneurysm: Unusual and Late Complication of Cardiac Surgery. Int J Clin Anesth Res. 2017; 1: 001-004. DOI: 10.29328/journal.ijcar.1001001
Copyright License: © 2017 Cottini M, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Pseudoaneurysm; Cardiac surgery complication; Pseudoaneurysm; Mitral valve replacement
Human Rights Statements and Informed Consent: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later revisions. Informed consent was obtained from the patient for being included in the study.
ABSTRACT
Despite the background of advances in cardiac surgery procedures for higher risk population, the postoperative complication has already been a challenge for cardiac surgeon and Heart-Team. Future perspectives to exceed this challenge could be periodically patient’s follow up and advance diagnostic workup. We describe the diagnosis of a large sub mitral left Ventricle Pseudoaneurysm that was identified in a 59-year-old woman 17 years after she underwent aortic and mitral valve replacement for rheumatic valvular disease.
INTRODUCTION
Left ventricle pseudoaneurysm (LVP) after cardiac surgery is uncommon and rare postoperative complication and its cause is not clear. The LVP could be defined sub aortic, sub mitral and intervalvular when developed in theinter annular zone between the mitral and aortic [1-4]. An early multimodality diagnostic workup up could reduce morbidity and mortality hence increasing related survival of the patients suffering of fibrosa LVP. We presented a 59-year-old woman with sub mitral left ventricle pseudoaneurysm occurring 17 years after cardiac surgery.
CASE REPORT
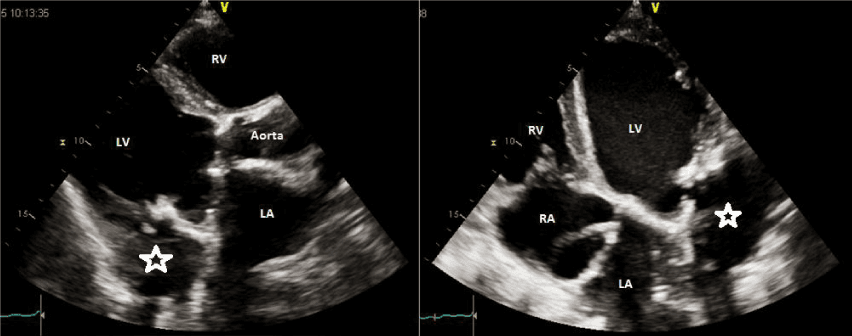
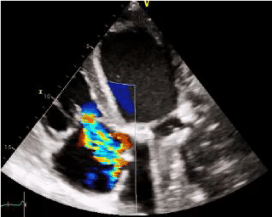
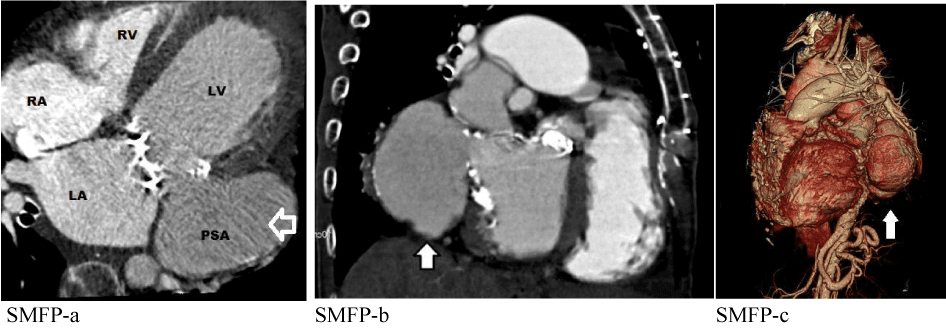
A 59-years-old Caucasian female was admitted to our Institution with dyspnea and asthenia since few months. Her cardiac history revealed successful mechanical aortic and mitral valve replacement. A standard transthoracic echocardiography (TTE) showed an echogenic image involving basal segment of lateral wall with defined edges adjacent to Left Ventricular Outflow Tract (LVOT) in the region of the mitral-aortic junction with no LVOT gradient and/or shunts, suggesting for sub mitral left ventricle pseudoaneurysm (Figure 1a,b). Left ventricle ejection fraction (LVEF) was 30%, the stroke volume (SV) 40 ml/beat, LV end diastolic volume (LVEDV) 182 ml, LV end sistolic volume (LVESV) 128 ml, sPAP 42 mmHg (Figure 1c). To better understand the relationship with the nearest cardiac structure, a Cardiac Multi Detector 64-slice Computed Tomography (MDCT-64) was performed demonstrating giant left ventricular pseudoaneurysm (10 × 6 cm) originating from the posterior mitral valve annulus (Figure 2). According to patient clinical history, the sub mitral LV pseudoaneurysm could be a late and rare cardiac surgery complication after the previous mitral valve replacement. Considering the clinical and diagnostic overview of the patient and the surgical risk, discussing the case to the Department Heart Team (Euro score II 9.02%, STS score risk of mortality 8,68%, Morbidity or mortality 35,48%, Prolonged Ventilation 23%), wesuggested the surgical intervention but the patient refused. She was regularly visited in the outpatient department and at the moment she was NYHA II.
Figure 1a,b: Standard transthoracic echocardiography showed an echogenic image involving basal segment of lateral wall adjacent to Left Ventricular Outflow Tract (LVOT) in the region of the mitral-aortic junction with no LVOT gradient and/or shunts, suggesting for sub mitral left ventricle (star). LV: left ventricle; LA: left atrium; RV: right ventricle; RA: right atrium.
Figure 1c: Transthoracic echocardiography Continuous wave-doppler showing a mild-moderate no mitral regurgitation but tricuspid regurgitation, no sign of heart failure, no pericardial effusion.
DISCUSSION AND CONCLUSION
The sub mitral LV pseudoaneurysm (SLVP) is a rare condition that has been reported as a result of endocarditis, chest trauma or heart valve surgery. Endocarditis and aortic valve surgery are the two most frequent causes (0.02-2% of cardiac surgery postoperative complications). The pathophysiology of SLVP is multiple: congenital (weak and thin membranous region in the sub-mitral location caudal to the postero-medial segment of the mural leaflet and adjacent aortic leaflet of the mitral); the infection such as endocarditis caused the distortion and thinning of mitral-aortic interventricular groove; previous heart surgery, in particularly mitral or aortic valve replacement, caused modification of heart geometry and tension on ventricle. Complications of SLVP include fistula formation, such as perforation with shunt of LVOT into the left atrium, infection, compression of the coronary or pulmonary arteries, also increase of size with the possibility of rupture, embolization, or primary valvular dysfunctions [3] (Table 1). Before therapeutic choice (medical, surgical or endovascular), it is important the identification of size, anatomical relationships and clinical patient’ details. The operative risk and the critical issues of the pathology might address the physician to the appropriate choice. In addition, the current multimodality imaging and the future perspective in diagnostic workup might get better the management of these patients, hence the short and long term-survival. We suggested to perform always multimodality workup in suspicious of post-cardiac surgery LV pseudoaneurysm to have the best imaging sensibility and specifity in diagnosis hence to have much more information for therapeutic choice and patient-safe.
| Table 1: Pseudoaneurysm features according to the scientific literature. IE: infective endocarditis; CS: cardiac surgery; MVR: mitral valve replacement; CD: congenital disease; BAV: bicuspid aortic valve; TTE: transthoracic echocardiogram; TEE: trans esophageal echocardiogram; LV: left ventricle; MRI: magnetic resonance image; CT: computed tomography; SLVP: sub mitral left ventricle pseudoaneurysm. | |
| Sub Mitral Fibrosa Pseudoaneurysm (SMF-P) features. | |
| First Reported | 1813 |
| Scientific literature review | Less than 100 described cases |
| Submitral | About the 70% |
| Pathophysiology | Chest trauma Post-infectious complications (such as IE) Myocardial infarction |
| Post CS complications | After resection of posterior mitral leaflet Extensive annular calcification Insertion of oversize prosthesis REDO MVR |
| Associated CD | Coartation of aorta BAV Anomalous pulmonary venous return |
| Most common presentation | Heart Failure Valve or prosthesis dysfunction |
| Echocardiogram (TTE or TEE) | Pseudoaneurysm details (size, orifice, etc.) Systolic and diastolic LV details |
| MRI or CT scan | Identify additional congenital anomalies Define SMF-P ZDFSVVVXVs |
| Complications of SMF-P | Perforation and shunt in near structure Compression of near structure Rupture early, delayed or late Embolization Valve disease |
| Treatment of choice | Surgery But endovascular and observational choices have to be considered according to clinical and imaging of the patient. |
The principal surgical approach is the median sternotomy and consequently the exclusion of SLVP by direct suture or patch positioning. According to O’ Flynn et al. [4] and Namboodiri et al. [5] the direct closure of the orifice located outside the LV and outside the LV under thoracotomy or could be the available, safe and less invasive approach to do. On the contrary, Honda and colleagues [6] supported the conventional approach and surgical technique by closing the pseudoaneurysm with a patch and the median sternotomy approach (Table 2). The early surgical repair is often the recommended treatment, in particularly if the heart failure is present and the pseudoaneurysm size is more than 3 cm in diameter (Table 2) in order to reduce the risk of rupture or infection or compression of neighboring structures. Therefore, the most incidence of SLVP are in patient with a clinical history of previous cardiac surgery, hence the less invasive approach could be the safer and the more effective. Moreover a new therapeutic choice is spreading against surgery: endovascular choice has been taking place more and more in daily clinical work according to clinical and diagnostic features of the patients. According to our experience, in case of low SLVP size, and/or no infectious sign (increasing of white blood cell, negative inflammation index such as C-reactive protein and pro calcitonin) and/or high operative risk, the conservative choice is better with a short follow up. On the other hand, the surgical choice excluded definitively the pathology and the risk of SLVP rupture but has got technical risk, peri- and postoperative risks. Moreover, in the perspective of the SLVP therapy we have a new reliable and wide spreading choice: endovascular.
Table 2: Description of SLVP therapeutic choices: surgical, endovascular and observational. |
|
Treatment choices |
Indications |
Surgical |
Presence of heart failure |
Endovascular |
Alternative to surgery |
Observational |
When there wasn't tissue thickening |
REFERENCES
- Sudhakar S, Sewani A, Agrawal M, Uretsky BF. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa (MAIVF): a comprehensive review. J Am Soc Echocardiogr. 2010; 23:1009-1018. Ref.: https://goo.gl/cefh1V
- Grimaldi A, Ho SY, Pozzoli A, Sora N, Taramasso M, et al. Pseudoaneurysm of mitral-aortic intervalvular fibrosa. Interact Cardiovasc Thorac Surg. 2011; 13: 142-147. Ref.: https://goo.gl/h9XK8x
- Entrikin DW, Shroff GS, Kon ND, Carr JJ. Pseudoaneurysm of the mitral-aortic intervalvular fibrosa: a delayed complication of aortic root replacement. J Cardiovasc Comput Tomogr. 2011; 5: 333-335. Ref.: https://goo.gl/xOsaIF
- O’Flynn E, Purkayastha S, Athanasiou T, Casula R. Repair of a giant left ventricular pseudoaneurysm in a Jehovah’s Witness. Asian Cardiovasc Thorac Ann. 2006; 14: 328-330. Ref.: https://goo.gl/SU6EfO
- Namboodiri N, Dora SK, Thomas B, Misra M. Sub annular left ventricular pseudoaneurysm following mitral valve replacement. J Cardiothoracic Surge. 2008, 3: 28 doi:10.1186/1749-8090-3-28. Ref.: https://goo.gl/YVMFeX
- Honda K, Okamura Y, Nishimura Y, Hayashi H. Patch Repair of a Giant Left Ventricular Pseudoaneurysm AfterMitral Valve Replacement. Ann Thorac Surg. 2011; 91: 1596-1597. Ref.: https://goo.gl/hWjq0d