More Information
Submitted: March 17, 2025 | Approved: April 01, 2025 | Published: April 02, 2025
How to cite this article: Das A. Anesthetic Management of a Patient with Left Ventricular Thrombus Posted for Emergency Laparotomy. Int J Clin Anesth Res. 2025; 9(1): 013-016. Available from:
https://dx.doi.org/10.29328/journal.ijcar.1001028
DOI: 10.29328/journal.ijcar.1001028
Copyright License: © 2025 Das A. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Anesthetic Management of a Patient with Left Ventricular Thrombus Posted for Emergency Laparotomy
Arpita Das*
St. Peter’s Medical College and Research Institute, India
*Address for Correspondence: Arpita Das, St. Peter’s Medical College and Research Institute, India, Email: [email protected]
Left ventricular thrombus (LVT) is a life threatening complication following acute coronary syndromes but in modern era its incidence has reduced since the introduction of primary percutaneous intervention. LVT is associated with higher morbidity and mortality due to its thromboembolic events and major adverse cardiac events (MACE). This is a case report of 30-year-old male who presented with acute abdomen and left ventricular thrombus. CECT abdomen revealed superior mesenteric artery (SMA) thrombosis and echocardiography revealed severe ventricular dysfunction (ejection fraction, EF<30%) with global hypokinesia and LVT. SMA thrombosis is fatal and if left unattended can lead to intestinal ischemia and gangrene, hence immediate intervention is warranted. This patient had undergone emergency laparotomy under general anesthesia for the resection of gangrenous jejunal segment with mucous fistula . This case report discusses perioperative management considerations in such cases.
Left ventricular thrombus is a morbid complication following acute coronary syndrome as well as in non-ischemic cardiac issues like dilated cardiomyopathy. Incidence of LVT varies from 4% to 39% [1], in different studies, depending on the timing and frequency of screening. In pre-percutaneous coronary intervention era incidence was around 28% - 46% which has reduced to 5% - 15% post PCI introduction [2]. LVT is predominantly seen in anterior wall MI with reduced ejection fraction, involvement of left anterior descending artery with large infarct or STEMI. There are reported cases of LVT in patients with non-ischemic cardiomyopathy due to severe left ventricular dysfunction. Incidence of LVT following cardiomyopathy is anywhere between 2% and 36% [3]. As per studies by Ching Li, et al. and McCarthy, et al. LVT causes cerebral and peripheral arterial embolism and subsequent mortality and have higher chances of major adverse cardiac events (MACE). Mortality associated with LVT ranges from 24% to 34%, with a higher mortality rate observed in the geriatric population. Treatment with antiplatelet and anticoagulation has shown benefits in reducing embolization incidence.
Superior mesenteric artery (SMA) thrombosis is one of the complication of LV thrombus. It is due to thromboembolic obstruction of the SMA, providing arterial supply to small intestine and ascending colon, regardless of underlying atherosclerotic diseases of the SMA. Thrombosis leads to ischemia followed by infarction of small bowel, more proximal the obstruction more severe and widespread the intestinal infarction. Early detection and management are crucial, there is a 50% chance of survival if diagnosed within 24 hrs. And, survival drops to 30% if diagnosed beyond 24 hrs. [4]. Definitive treatment is essential upon diagnosis, with treatment options of minimally invasive catheter directed thrombolytic infusion to mesenteric bypass. However, if signs of peritonitis are present , then on the basis of strong suspicion of bowel gangrene, laparotomy should be done.
In patients with mesenteric ischemia presented with LV clot, mortality risk doubles considering the urgency of surgery and limited time for the commencement of therapeutic anticoagulation.
A 30 year old male, weight approximately 50 kg was admitted to intensive care with complaints of intermittent abdominal pain for past 3 weeks associated with vomiting and diarrhea with contrast enhanced computed tomography (CECT) reported as SMA thrombosis. He also complained of dyspnea on exertion (NYHA class III) for past 3 weeks. He was recently diagnosed (1 month ago) with hypertension but not on medications. He gave negative history of any possible cardiac ailments, lower respiratory tract infections, chronic kidney disease, and addiction or drug allergies. There was no family history of cardiac issues, diabetes or hypertension. On examination, Patient’s vital signs were within normal limits, with no tachycardia, hypotension, pallor, cyanosis, or pedal edema. Airway was Mallampatti grade 3, with 3- finger mouth opening and normal range of neck extension. On auscultation, lungs were clear with Broncho vesicular breath sounds; S1, S2 heard without murmur. Abdomen was mildly distended with tenderness, guarding and rigidity on palpation; and bowel sounds were absent.
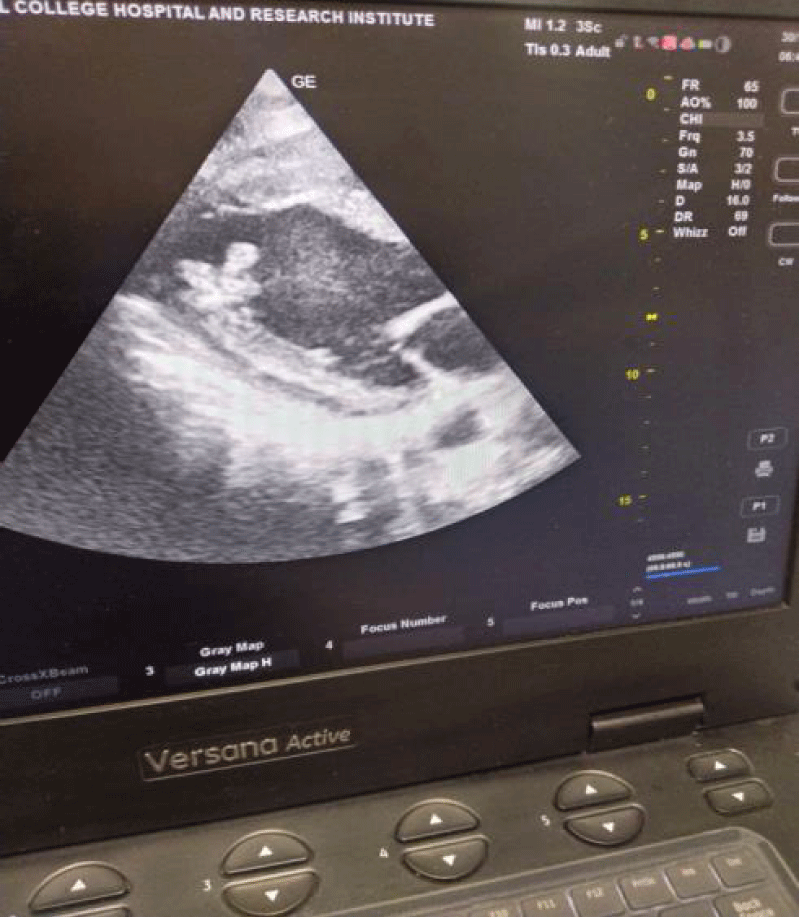
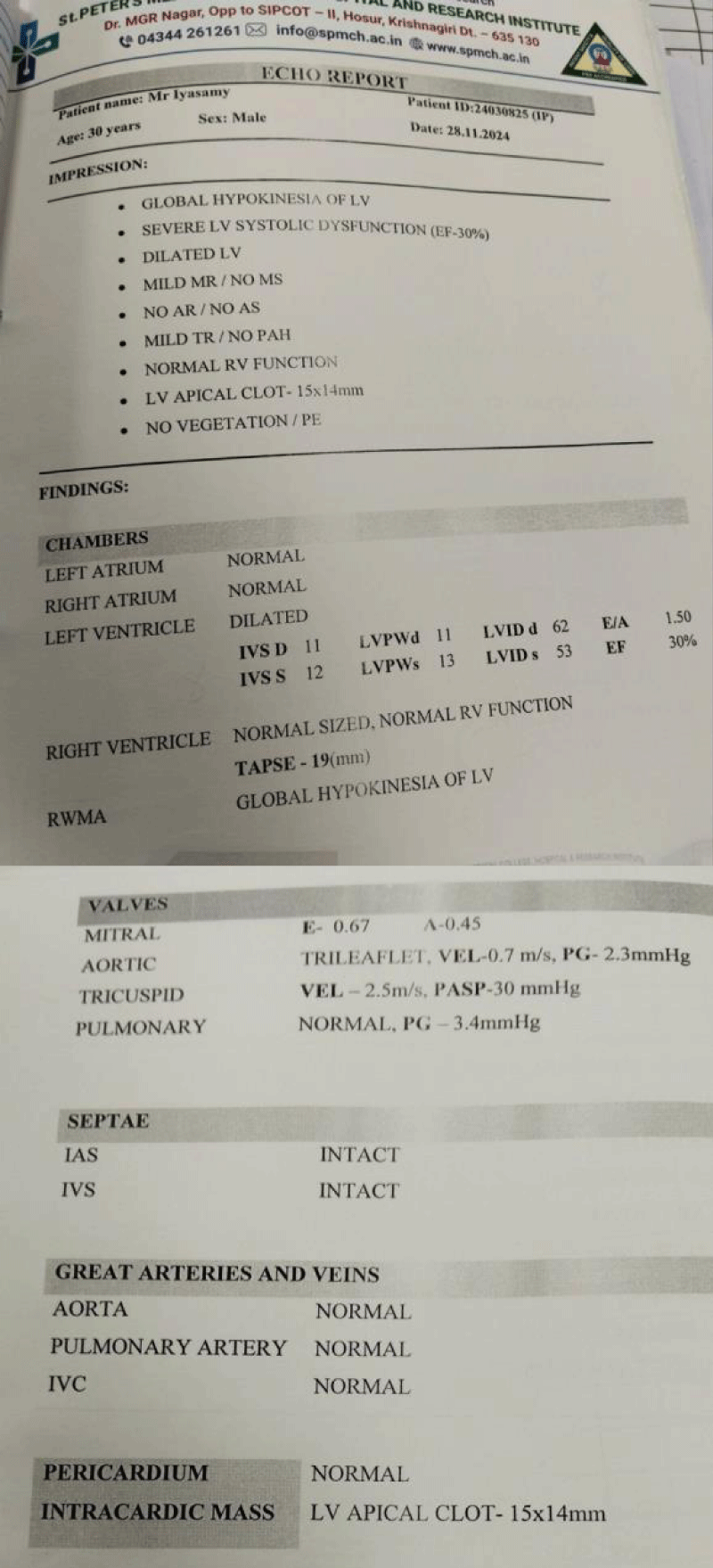
Routine blood parameters were all within normal limits, serial arterial blood gas analysis were normal with lactate level around 1.0. Electrocardiograph showed T wave inversion in all leads and left ventricular hypertrophy. Echocardiography done in view of NYHA class 3 dyspnea and ECG changes, revealed global hypokinesia of left ventricle with severe LV systolic dysfunction and ejection fraction (EF=30%); and LV apical clot present (Figures 1,2). Serial Troponin-I level done at 0 and 6hr and result was less than 0.01. Cardiologist opinion was taken for poor LV function and LV apical clot and as advised patient was started on Injection Heparin infusion at 18 unit/kg after loading dose of 5000units, targeting activated Partial Thromboplastin Time (aPTT) between 50-70 with monitoring done every 8th hrly; dual antiplatelet Ecosprin and Clopidogrel 75 mg each with atorvastatin, Ivabrad and furosemide injection started . Patient was taken up for emergency laparotomy and high risk for anesthesia and chances of intraoperative arrhythmia or cardiac arrest or postoperative stroke with need for postoperative ventilation and ICU stay explained to patient relatives.
Figure 1: In parasternal long axis view of the Left ventricular chamber, thrombus is seen protruding into the cavity and is mobile and pedunculated. Indicated by red arrow.
Figure 2: 2D Echocardiography report.
Preoperative aPTT was 47.7 seconds while on continuous heparin infusion. 2 units of packed red blood cells, 4 units of Fresh frozen plasma and platelets each reserved for intraoperative use if required. Heparin infusion stopped 1 her prior taking up for surgery. Prior to induction of general anesthesia connected. Vital signs were within normal limit without any vasopressor and ionotropes support. Premedication Inj. Fentanyl 200 µg and Inj. Midazolam 2 mg intravenously (I.V) given. After performing Allen’s test, under local anesthesia and aseptic precautions right radial artery was cannulated with a 20-gauge catheter for arterial blood pressure monitoring. Under local anesthesia and aseptic precautions, using ultrasound, right internal jugular vein cannulated with triple lumen 7 Fr central venous catheter. After preoxygenation with 100% oxygen, induction done with Inj. Etomidate 10 mg i.v and Inj. Atracurium 25 mg i.v. Intubated with cuffed endotracheal tube size 8.0. Positioning of tube confirmed with capnogram. Patient kept on controlled ventilation with continuous end tidal CO2 monitoring, anesthesia was maintained with oxygen, nitrous oxide, and isoflurane with MAC between 0.5 and 1.0. Relaxation maintained with Inj. Atracurium 5 mg and for analgesia Inj. Paracetamol 1 mg was given I. V and Inj. fentanyl was repeated 25 µg every hour. Antibiotics Inj. Piperacillin-Tazobactam 4.5 gm and Inj. metronidazole 500 mg was given. Inj. Protamine 50 mg was given to reverse residual heparin before incision. Intraoperative surgical findings were gangrenous change in the mid jejunal segment 25 cm from the DJ flexure for about 70 cm. Proceeded with resection of gangrenous mid jejunal upto proximal ileum. Proximal end of jejunum kept as stoma at left hypochondriac and distal end ileum is kept as stoma at right iliac region. Surgery went uneventful for 2 hrs with approximately 200 ml blood loss and 250 ml urine output. The patient was started on norepinephrine support at 2 µg/min IV infusion and 1.2 liters of intravenous fluids were administered. In postoperative period patient was shifted to ICU with Endotracheal tube (ET) tube on ventilator and heparin infusion restarted at 1200U/hr. maintaining target aPTT. Twenty-four hours post-surgery, vasopressor support tapered off to minimal and patient extubation was done, Post extubation, the patient’s vital signs remained stable. On postoperative day 4 patient was shifted to ward on antiplatelets and anticoagulants without any complications.
Acute mesenteric ischemia (AMI) is due to interrupted blood supply to the intestine, leading to intestinal necrosis, severe metabolic derangements and patient death if untreated. The overall incidence is low (0.09% - 0.2% of all acute admissions to emergency departments), but a common cause of emergent intestinal resection [5]. It causes significant mortality, however early diagnosis and intervention can save life. Half of cases of AMI are due to acute SMA embolism [6]. Mesenteric emboli can originate from valvular disorders, atrial fibrillation or severe left ventricular dysfunction. Delay in diagnosis is the dominant factor that accounts for high mortality rates of 30% - 70% despite increased knowledge of this entity. Every 6h of delay in diagnosis (actually-delay in CT Angiogram) doubles mortality [7].
Left ventricular thrombus (LVT) is reported after acute myocardial infarction (MI) and even in non-ischemic cardiomyopathies. Historically, the incidence of LVT following acute MI had been reported to be 20% - 40%, and even 60% among patients with large anterior MI [8]. Post introduction of thrombolytic therapy for MI, the incidence of LVT was reduced, mostly due to its use in reducing wall motion abnormalities. STEMI patients have been reported to be more likely to have LVT compared to non-STEMIs (43.1% vs. 5.0%). Across studies, described predictors of LVT are anterior MI/left anterior descending territory, involvement of left ventricular (LV) apex regardless of the coronary artery affected, akinesis or dyskinesis, reduced LVEF and large infarct size [9]. In this case report, patient complained of chest pain and shortness of breath for past 3 weeks, however patient didn’t seek medical treatment. On arrival, echocardiography revealed regional wall motion abnormality with reduced left ventricular ejection fraction, indicating that patient might have had acute coronary syndrome. Most LVT cases are due to ischemic cardiomyopathy but few due to non-ischemic cardiomyopathies were also reported. Previous studies reported prevalence of LVT up to 36% in the setting of dilated cardiomyopathy, with an incidence of 11% for embolic events. The reported risk of embolic events from a LVT post-MI ranges from 6.1% to 86% and seems to be greatest in the first 3 months after MI [10]. Multiple studies have demonstrated that thrombus characteristics associated with systemic embolism include protrusion into the LV cavity, mobility independent of myocardium, patient age > 68, thrombus area, length of the thrombi in the lumen, and LVT recurrence [11].
For our patient, the echocardiography report do not mention about the characteristic of the clot except it’s size, but in image the clot is seen to be pedunculated and protruding into the cavity hence, more prone for embolism.
Thus, mesenteric ischemia is usually a sequel thrombo-embolic complication of left ventricular thrombosis and is not a separate entity. As an etiology of acute mesenteric ischemia, it is mostly due to embolic complication of reduced left ventricular ejection fraction with or without left ventricular thrombosis. Hence, supposed incidence of such cases are more, however no studies mentioning such cases have been reported in the literature. Arikrishnan, et al. [12] reported a rare case of left ventricular thrombus posted for emergency decompression craniectomy done under general anesthesia and Balasubramaniam, et al. reported a case of traumatic foot ulcer in patient with reduced EF and LVT for skin grafting under peripheral nerve blocks.
Left ventricular thrombosis is treated with prolonged (at least 3 months) anticoagulation with dual antiplatelet therapy, surgical thrombectomy is an alternative option but with higher morbidity and mortality risk. For our patient, surgery was emergency hence patient was started on heparin infusion which will help both in left ventricular thrombosis as well as in mesenteric ischemia.
Fluid resuscitation is essential for the management of the patient with suspected AMI.
Patients with AMI often exhibit severe metabolic and electrolyte disturbances due to underlying bowel infraction and reperfusion, which needs correction with fluid resuscitation and respective electrolyte correction in preoperative period. To guide resuscitation and prevent cardiovascular collapse during anesthesia induction, adequate hemodynamic monitoring need to be ensured.
The intraoperative plan aimed to avoid tachycardia and hypotension, maintain preload and systemic vascular resistance. Hence opioid based induction with etomidate was used and preinduction invasive arterial line was inserted for hemodynamically monitoring.
The general principle to avoid clot embolisation is to avoid hyperdynamic heart by avoiding tachycardia, arrhythmia and increased contractility. Use of inotropes and vasopressors was minimized to avoid clot embolization and as it may compromise mesenteric blood supply to the bowel, respectively. Intraoperative use of local anesthetic with adrenaline is avoided. Maintaining adequate intravascular volume is essential as patient cannot tolerate both hypo and hypervolemia due to restricted LV cavity size despite adequate ventricular dilatation. For maintenance opioid and inhalation anesthetic used isoflurane with a MAC < 1.0. In postoperative period anticoagulation need to be continued and oral anticoagulants were initiated alongside dual antiplatelet therapy.
This is a rare and complex case due to the nature of illness and urgency of surgery. Though the patient successfully underwent intraoperative and postoperative period without any events, but he requires long-term anticoagulation therapy with regular follow-up. Left ventricular thrombosis has got lifelong risk of systemic embolism and its sequelae.
Left ventricular thrombosis presenting for non-cardiac emergency surgery is a complex case. Life-threatening complications with high incidence of mortality is a possibility. Such cases require pre-operative fluid resuscitation in a judicious way requiring intraoperative hemodynamic monitoring and real-time fluid assessment. Drugs causing cardiac depression and ionotropes causing hyperdynamic heart to be avoided. Even postoperative and anticoagulation should be continued at discharge and are at lifelong risk of systemic embolism or its complications such as mesenteric or limb ischemia.
- Habash F, Vallurupalli S. Challenges in management of left ventricular thrombus. Ther Adv Cardiovasc Dis. 2017;11:203-213. Available from: https://doi.org/10.1177/1753944717711139
- Robinson AA, Jain A, Gentry M, McNamara RL. Left ventricular thrombi after STEMI in the primary PCI era: a systematic review and meta-analysis. Int J Cardiol. 2016;221:554-559. Available from: https://doi.org/10.1016/j.ijcard.2016.07.069
- Gottdiener JS, Gay JA, VanVoorhees L, DiBianco R, Fletcher RD. Frequency and embolic potential of left ventricular thrombus in dilated cardiomyopathy: assessment by 2-dimensional echocardiography. Am J Cardiol. 1983;52:1281-1285. Available from: https://doi.org/10.1016/0002-9149(83)90588-x
- Boley SJ, Feinstein FR, Sammartano R, Brandt LJ, Sprayregen S. New concepts in the management of emboli of the superior mesenteric artery. Surg Gynecol Obstet. 1981;153(4):561-569. Available from: https://pubmed.ncbi.nlm.nih.gov/7280946/
- Duran M, Pohl E, Grabitz K, Schelzig H, Sagban TA, Simon F. The importance of open emergency surgery in the treatment of acute mesenteric ischemia. World J Emerg Surg. 2015;10:45. Available from: https://doi.org/10.1186/s13017-015-0041-6
- Clair DG, Beach JM. Mesenteric ischemia. N Engl J Med. 2016;374:959-968. Available from: https://doi.org/10.1056/nejmra1503884
- Kassahun WT, Schulz T, Richter O, Hauss J. Unchanged high mortality rates from acute occlusive intestinal ischemia: six-year review. Langenbecks Arch Surg. 2008;393:163-171. Available from: https://doi.org/10.1007/s00423-007-0263-5
- Keeley EC, Hillis LD. Left ventricular mural thrombus after acute myocardial infarction. Clin Cardiol. 1996;19:83-86. Available from: https://doi.org/10.1002/clc.4960190203
- Meurin P, Carreira VB, Dumaine R, Shqueir A, Milleron O, Safar B, et al. Incidence, diagnostic methods, and evolution of left ventricular thrombus in patients with anterior myocardial infarction and low left ventricular ejection fraction: a prospective multicenter study. Am Heart J. 2015;170:256-262. Available from: https://doi.org/10.1016/j.ahj.2015.04.029
- Vaitkus PT, Barnathan ES. Embolic potential, prevention, and management of mural thrombus complicating anterior myocardial infarction: a meta-analysis. J Am Coll Cardiol. 1993;22:1004-1009. Available from: https://doi.org/10.1016/0735-1097(93)90409-t
- Leow AS, Sia CH, Tan BY, Kaur R, Yeo TC, Chan MY, et al. Characterization of acute ischemic stroke in patients with left ventricular thrombi after myocardial infarction. J Thromb Thrombolysis. 2019;48:158-166. Available from: https://doi.org/10.1007/s11239-019-01829-6
- Arikrishnan T, Chakravarthy D, Uthaman D, Srinivasan G. Rare case of left ventricular thrombus post-myocardial infarction for emergency decompressive craniectomy. J Neuroanaesthesiol Crit Care. 2022;9:112-114. Available from: http://dx.doi.org/10.1055/s-0041-1734421